Abstract
Objective: To study the cardiotocographic (CTG) changes and perinatal outcome of epidural analgesia with ropivacaine in labor. Material and methods: Sixty women with term pregnancy in active labor were randomly distributed in two equal groups. Epidural group received 10 mL of 0.1% Ropivacaine as a bolus with top up doses according to parturient’s demand. Control group did not receive epidural analgesia. Maternal monitoring and continuous fetal monitoring with CTG were done. Neonatal outcome was evaluated. Results: The mean time for onset of analgesia after first dose of epidural ropivacaine was 13.13 minutes. The visual analogue scale (VAS) score was reduced from 74.57 mm to 13.5 mm at 30 min and 17.17 mm at 1 hour providing significant pain relief (p <0.05). About 83.3% cases had normal CTG tracing and 16.7% had suspicious tracing with ropivacaine which was comparable to 90% and 10% respectively in control group. Instrumental delivery or cesarean section rate did not increase with ropivacaine. There was no significant difference in birth weight, Apgar score, intensive care admission or neonatal mortality. Patient satisfaction rate with epidural ropivacaine was excellent in 53% cases. Conclusion: Epidural analgesia with ropivacaine provides rapid and significant pain relief during labor and is efficacious and safe for the mother as well as the baby.
Keywords: Cardiotocography, epidural analgesia, labor analgesia, ropivacaine
“The delivery of the infant into the arms of a conscious and pain free mother is
one of the most exciting and rewarding moments in medicine”.
– Moir DD
Labor analgesia has come a long way in providing pain relief to women during labor, so that the experience remains just a wonderful memory of the birth of their child. Epidural analgesia is commonly used for this purpose.
Bupivacaine is a commonly used local anesthetic for labor epidural analgesia. Ropivacaine is a new long-acting amide closely related structurally to bupivacaine and mepivacaine, with a similar potency and duration.1 Ropivacaine has the advantage of having a lower neurotoxic and cardiotoxic potential and less intense motor block than bupivacaine;2 thus reducing the instrument delivery rate and cesarean delivery rate with ropivacaine.1,3 These properties make ropivacaine a desirable local anesthetic agent for obstetrical analgesia.
Cardiotocography (CTG) is a dynamic test for the state of oxygenation of fetus during labor. There is controversy whether epidural analgesia affects the maternal and fetal outcomes and progress of labor. Baseline fetal heart rate (FHR) variability has become an important parameter in the diagnosis of fetal distress when electronically monitoring the fetus. Loss of this baseline variability has been noted to be associated with fetal distress, and in association with late deceleration or severe variable deceleration patterns has been shown to be ominous. However, it can also be modified by prematurity and the administration of certain drugs to the mother.4
Objective
The objectives of this study were to assess the CTG changes and perinatal outcome with ropivacaine for epidural analgesia in labor.
MATERIAL AND METHODS
This is a prospective randomized study, conducted in the Dept. of Obstetrics and Gynecology, Sarojini Naidu Medical College, Agra, Uttar Pradesh. Sixty women with term pregnancy (37-41 weeks), in active labor were selected for this study. They were randomly distributed in two groups of 30 each. Group A (Epidural group) received epidural ropivacaine for pain relief and Group B (Control group) did not receive epidural analgesia.
The inclusion criteria were – singleton pregnancy with vertex presentation, reactive nonstress test and established active stage of labor (uterine contractions 2 per 10 minutes, lasting 30 to 40 seconds and cervical dilatation ≥3 cm). Both primipara and multipara were included. The exclusion criteria were women with malpresentations, cephalopelvic disproportion, previous cesarean delivery, antepartum hemorrhage, medical complications like diabetes, hypertension or asthma and neurological conditions where rise in intracranial tension during delivery is anticipated (meningitis, cancer, brain abscess, brain hemorrhage, head injury).
Informed consent was taken. After prehydrating the women in Group A with 500 mL of ringer lactate solution, the epidural catheter was put in place and 10 mL of 0.1% ropivacaine was injected as a bolus dose. The top up doses were given according to parturient’s demand.
After each dose, maternal vitals (blood pressure, pulse rate and respiratory rate) were monitored every 5 minutes for the next 20 minutes and then every 30 minutes till delivery. Degree of pain relief was evaluated with a visual analogue scale (VAS) score before the first epidural injection and at 30 minutes and 1 hour. CTG tracing was taken at the time of admission and was repeated half hourly in the first stage and continuously in the second stage for fetal monitoring. Baseline fetal heart rate, beat-to-beat variability, accelerations and decelerations were noted and the CTG was classified as normal, suspicious or pathological as per the National Institute for Health and Care Excellence (NICE) guidelines.5 A partogram was maintained to assess the progress of labor. The effect of drug on neonatal outcome was studied by observing Apgar score at 1, 5 and 10 minutes after birth, birth weight, Neonatal Intensive Care Unit (NICU) admission and neonatal mortality. Maternal side effects were noted. The analgesic effect and patient satisfaction was assessed after 24 hours and rated as excellent, good, average and poor.
The student t test and Chi-square test were applied and p value calculated. A p value <0.05 was considered statistically significant.
OBSERVATION AND RESULTS
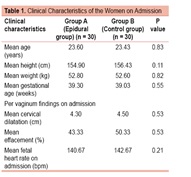
The clinical characteristics of the epidural group and control group are given in Table 1. In both the groups, most of the women were primiparas (60% in epidural group and 73.3% in control group). Both the groups were also comparable in terms of age, height, weight and period of gestation of the women at the time of admission.
Per vaginum findings at the time of admission were also similar in both the groups. Mean cervical dilatation was 4.30 cm in epidural group and 4.50 cm in control group, with mean effacement of 43.33% and 50.33% respectively (Table 1). Head was at -1 station in most of the parturients in both groups and membranes were intact in all of them.
The mean time for onset of analgesia after first dose of epidural ropivacaine was 13.13 minutes. The VAS score was 74.57 mm before giving epidural analgesia and 13.5 mm at 30 minutes and 17.17 mm at 1 hour after giving epidural ropivacaine. Thus the degree of pain relief was statistically significant (p <0.05). In the epidural group, 14 women (46.67%) needed 2 top up doses, while 12 (40%) needed one and 3 women (10%) needed 3 top up doses. One woman did not need any top up dose.
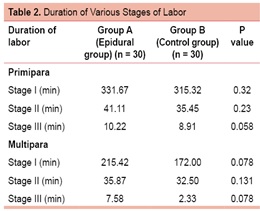
As shown in Table 2, the mean duration of first and second stages of labor were slightly prolonged in the epidural group than the control group but the difference was not statistically significant.
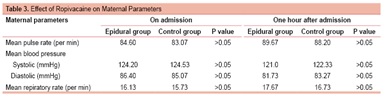
Maternal parameters (pulse rate, blood pressure, respiratory rate) at the time of admission and 1 hour after receiving epidural ropivacaine showed no significant changes in either group (Table 3).
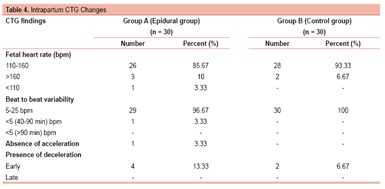
During intrapartum CTG monitoring in epidural group, fetal heart rate was normal (110-160) in 85.67% cases, tachycardia (>160 bpm) was seen in 10% cases and bradycardia (<110 bpm) in only 1 case (3.33%). In the control group, fetal heart rate was normal in 93.3% cases, tachycardia was seen in 6.67% cases with no case of bradycardia. In the epidural group, beat-to-beat variability was normal (5-25 bpm) in 96.67% cases and <5 bpm (for 40-90 min) in one case, while in the control group all cases had normal beat-to-beat variability. In the epidural group, there was absence of acceleration in 1 case (3.33%) and early decelerations in 4 cases (13.3%). In the control group, none of the cases showed absence of acceleration while early decelerations were seen in 2 cases (6.67%). None of the women in either group had late decelerations. (Table 4)
In the study, 83.3% cases in the epidural group and 90% in the control group had normal CTG tracing during intrapartum period, while suspicious CTG findings were found in 16.7% and 10% respectively. The difference was not significant. None of the cases had pathological CTG tracings.
The mode of delivery was vaginal in maximum women in both the groups (26/30 [87.7%] vs 28/30 [93.3%]). In epidural group, forceps were applied in one case (3.33%) because of nonrotation of head, while none of the cases in the control group were delivered instrumentally. Three women (10%) in the epidural group had cesarean section because of nonprogression of labor or fetal distress, while two women (6.67%) in the control group had cesarean section for nonprogression of labor. Thus there was no statistically significant increase in rate of instrumental delivery or cesarean section rate with epidural ropivacaine (p = 0.53)
(Fig. 1).
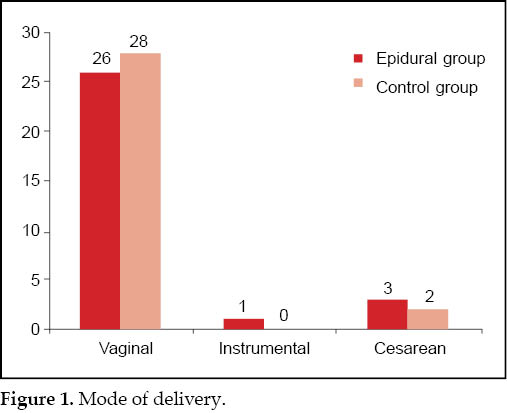
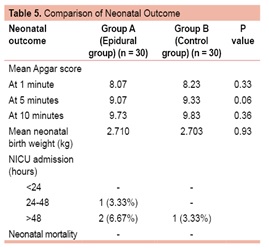
Table 5 compares the neonatal outcome in the two groups. Eighty percent cases in epidural group and 67% cases in control group had birth weight between 2.5-3 kg. There was no statistically significant difference in the mean birth weight in both the groups (2.710 kg in epidural group and 2.703 kg in control group; p = 0.93). Mean Apgar score at 1 minute in epidural group was 8.07 and in control group was 8.23. Mean Apgar score at 5 minutes was 9.07 and 9.33 respectively, while at 10 minutes it was 9.73 and 9.83 in the epidural and control group respectively. The difference in the two groups was not statistically significant. Three neonates (10%) in the epidural group and one (3.33%) in control group required NICU admission due to physiological jaundice and were discharged after conservative treatment. On statistically analyzing the difference it was found not significant. There was no neonatal mortality in either group.
Most evident maternal side effects with epidural ropivacaine were nausea, vomiting, headache and backache, occurring in <50% women. None of them were serious enough to discontinue the therapy. Overall patient satisfaction rate with epidural ropivacaine was excellent in 16 cases (53%), good in 9 (30%) and average in 5 cases (17%).
DISCUSSION
Use of epidural analgesia has gained popularity in many obstetric clinics. Ropivacaine and bupivacaine are the most commonly used local anesthetic agents in labor analgesia. Ropivacaine can be administered at various doses either as a bolus or as continuous epidural infusion. In our study we have administered 10 mL of 0.1% ropivacaine as bolus dose and top up doses on parturient’s request.
The mean time of onset of analgesic action after epidural ropivacaine in our study was 13.13 minutes, which is comparable to 12.8 minutes found by Clement et al,6 and 10 minutes by Stienstra et al.7
The degree of pain relief, measured by VAS at 0 and 30 minutes was 74.57 mm and 13.5 mm, which was comparable to other studies.6-8 The duration of first stage of labor varies from 276 to 545 minutes and that of second stage from 63 to 102 minutes in various studies.8-10 In our study it was 331.67 minutes and 41.11 minutes respectively.
The outcome of labor was not significantly affected and incidence of cesarean section and instrumental delivery was not increased by use of epidural ropivacaine. The cesarean section rate of our study (10%) was comparable to 10.2% cesarean rate of Litwin et al.1 The cesarean section rate varies from 5 to 20%8,9 and instrumental delivery rate varies from 1.66 to 32%1,8,9 in different studies.
Overall patient satisfaction rate was excellent in 53% and good in 30% women in our study. Similar results were seen by Mousa et al,9 i.e, excellent patient satisfaction rate in 65% cases and good in 55% cases. According to Lee et al,10 good/excellent satisfaction rate was 95% which was similar to our study (83%).
In the present study, 1 case (3.33%) had fetal bradycardia with ropivacaine while 4 (13.33%) had early decelerations and 1 (3.33%) had decreased variability. Owen et al8 had one case of fetal bradycardia which was managed by ephedrine.
Tugrul et al11 found no significant change in terms of neonatal breathing rate, umbilical cord CO2, O2, pH levels and Apgar scores with 0.2% ropivacaine. The present study also did not find any significant adverse effect on neonatal outcome with ropivacaine.
A variety of changes of fetal heart rate parameters have been attributed to epidural anesthesia. Epidural anesthesia with lidocaine may cause tachycardia in a small percentage of women and decreased fetal heart rate variability in others. No changes in baseline fetal heart rate have been observed after epidural anesthesia with bupivacaine. The fetal heart rate changes with ropivacaine are still being studied. In general, epidural anesthesia in the absence of maternal hypotension or uterine hypertonus causes minimal changes in the fetal heart rate parameters. Those changes that do occur are neither universal nor predictable. Therefore, any alteration in fetal heart rate monitoring parameters occurring in a patient receiving epidural anesthesia should be evaluated and acted upon in the same fashion and by the same methods one would employ if the patient was not receiving epidural anesthesia.12
CONCLUSION
From our study, we can conclude that epidural analgesia with ropivacaine provides rapid and significant pain relief. It provides excellent patient satisfaction during labor with no major side effects. The duration of various stages of labor are slightly longer with epidural analgesia but it is not significant enough to alter the final outcome of labor. There is no increase in cesarean sections or instrumental delivery rate. There is no adverse effect on maternal parameters, intrapartum CTG findings or perinatal outcome. Thus the use of ropivacaine in epidural labor analgesia is efficacious and safe for the mother as well as the baby.
REFERENCES
- Litwin AA. Mode of delivery following labor epidural analgesia: influence of ropivacaine and bupivacaine. AANA J. 2001;69(4):259-61.
- Merson N. A comparison of motor block between ropivacaine and bupivacaine for continuous labor epidural analgesia. AANA J. 2001;69(1):54-8.
- Writer WD, Stienstra R, Eddleston JM, Gatt SP, Griffin R, Gutsche BB, et al. Neonatal outcome and mode of delivery after epidural analgesia for labor with ropivacaine and bupivacaine: a prospective meta-analysis. Br J Anaesth. 1998;81(5):713-7.
- Boehm FH, Woodruff LF, Jr Growdon JH. The effect of lumbar epidural anesthesia on fetal heart rate baseline Anesth Analg. 1975;54(6):779-82.
- National Institute for Clinical Excellence. The use of electronic fetal monitoring. Inherited Clinical Guideline C. 2001:p.1-27.
- Clément HJ, Caruso L, Lopez F, Broisin F, Blanc-Jouvan M, Derré-Brunet E, et al. Epidural analgesia with 0.15% ropivacaine plus sufentanil 0.5 microgram ml-1 versus 0.10% bupivacaine plus sufentanil 0.5 microgram ml-1: a double-blind comparison during labor. Br J Anaesth. 2002;88(6):809-13.
- Stienstra R, Jonker TA, Bourdrez P, Kuijpers JC, van Kleef JW, Lundberg U. Ropivacaine 0.25% versus bupivacaine 0.25% for continuous epidural analgesia in labor: a double-blind comparison. Anesth Analg. 1995;80(2):285-9.
- Owen MD, Thomas JA, Smith T, Harris LC, D’Angelo R. Ropivacaine 0.075% and bupivacaine 0.075% with fentanyl 2 microg/mL are equivalent for labor epidural analgesia. Anesth Analg. 2002;94(1):179-83.
- Mousa WF, Al-Metwalli RR, Mostafa M. Epidural analgesia during labor--0.5% lidocaine with fentanyl vs. 0.08% ropivacaine with fentanyl. Middle East J Anesthesiol. 2010;20(4):521-7.
- Lee BB, Ngan Kee WD, Ng FF, Lau TK, Wong EL. Epidural infusions of ropivacaine and bupivacaine for labor analgesia: a randomized, double-blind study of obstetric outcome. Anesth Analg. 2004;98(4):1145-52.
- Tugrul S, Oral O, Bakacak M, Uslu H, Pekin O. Effects of epidural analgesia using ropivacaine on the mother and the newborn during labor. Saudi Med J. 2006;27(12):1853-8.
- Lavin JP. The effects of epidural anesthesia on electronic fetal heart rate monitoring. Clin Perinatol. 1982;9(1):55-62.