Abstract
Systemic lupus erythematous (SLE) flaring-up as acute spinal subarachnoid hemorrhage (SSH) with acute transverse myelitis at conus medullaris is a rare presentation among various demyelinating syndromes in SLE. Presented here is the case of a 47-year-old female, a known case of SLE, who presented with sudden back pain with abrupt onset of symmetrical weakness of both lower limbs and double incontinence. MRI showed subarachnoid bleed with associated myelitis. The patient was treated with prednisolone, mycophenolate mofetil and hydroxychloroquine and has been without any flares for 18 months.
Keywords: Systemic lupus erythematosus, spinal subarachnoid hemorrhage, acute transverse myelitis
Diagnosis of demyelinating syndrome in systemic lupus erythematous (SLE) is made after careful exclusion of primary demyelinating diseases, infectious diseases, neoplastic and paraneoplastic syndromes. As per Chessa et al, various patterns of demyelinating syndrome observed in SLE are: i) neuromyelitis optica (NMO), ii) neuromyelitis optica spectrum disorders (NMOSD), iii) demyelinating syndrome prominently involving the brain (DSB), iv) demyelinating syndrome prominently involving the brainstem (DSBS) and v) clinically isolated syndrome.1 SLE flaring-up as acute spinal subarachnoid hemorrhage (SSH) with acute transverse myelitis at conus medullaris is a rare presentation among various demyelinating syndromes in SLE.
CASE REPORT
A 47-year-old female, a known case of SLE, presented with sudden back pain with abrupt onset of symmetrical weakness of both lower limbs and double incontinence. On motor system examination, patient had symmetrical spastic paraparesis (3/5) below L1, brisk deep tendon reflexes (DTR) with bilateral extensor plantar reflex. Sensory examination revealed reduced touch sensation with perianal pain and temperature loss. Autonomic system had bowel and bladder incontinence. Clinically, the deficit was localized to spinal cord at conus medullaris. Magnetic resonance imaging (MRI) whole spine study showed an extra-axial T1 hyperintense and T2 hypointense well-defined subarachnoid bleed noted from D1 with mild compression on dorsal aspect of spinal cord at D8 vertebral level along with subtle long segment signal changes at the conus medullaris, suggestive of associated myelitis (Figs. 1 and 2).
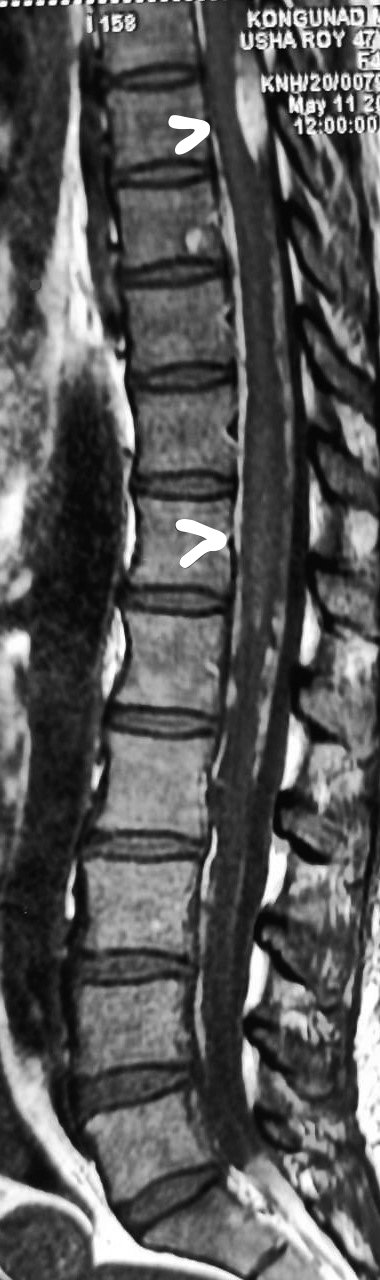
Figure 1. T1 Fat suppression image showing subarachnoid bleed with myelitis at D11, D12 level.
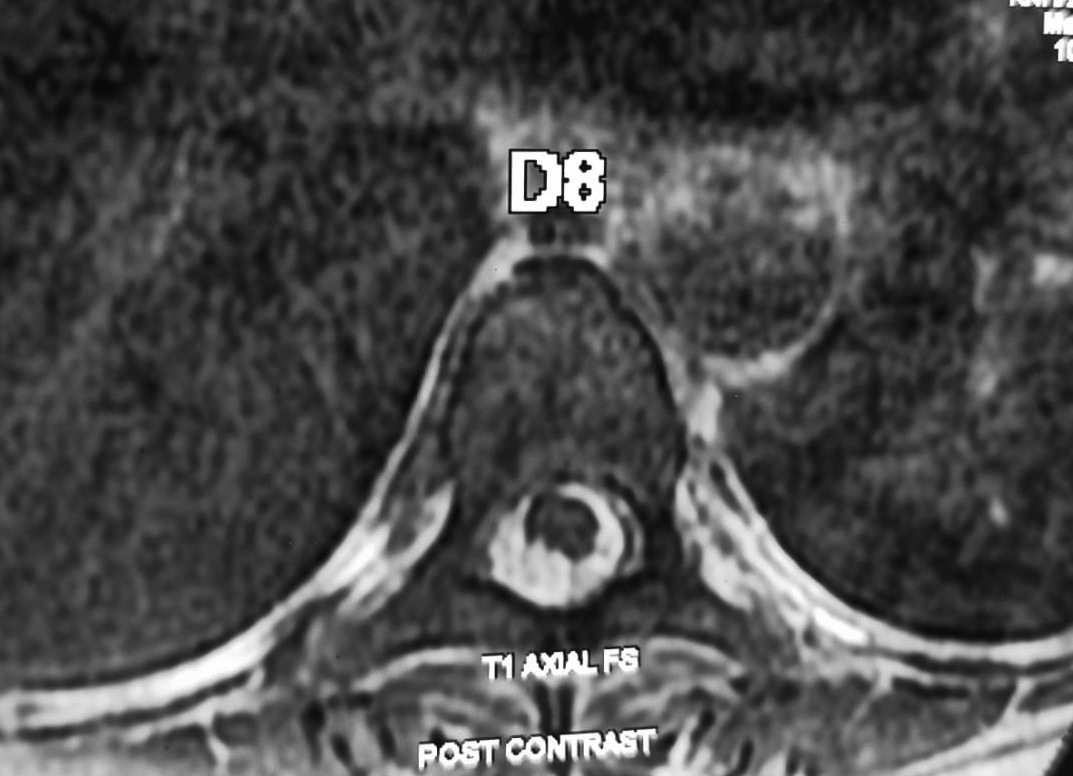
Figure 2. Post contrast axial view at D8 level showing cord compression at posterior aspect.
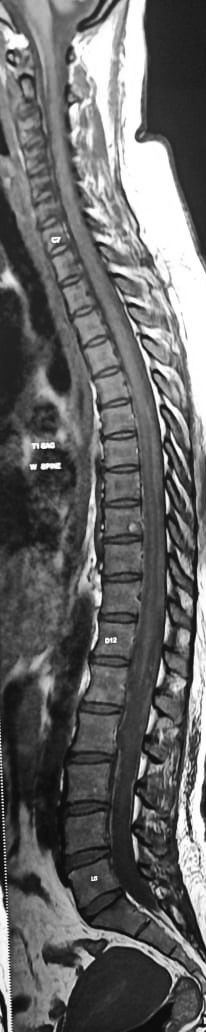
Figure 3. Reduction of SSH and myelitis.
MRI brain screening including optic nerves was normal. MR angiogram (MRA) with contrast showed normal intracranial and spinal vessels. Complete blood counts, coagualtion profile and serum complement levels were normal. Cerebrospinal fluid (CSF) analysis showed predominant lymphocytic inflammatory picture with xanthochromia (Total cells 100; polymorphs 40/mm3, lymphocytes 60/mm3); CSF biochemistry showed decreased glucose (38 mg/dL), raised protein (113 mg/dL) and no oligoclonal bands. Gram-staining acid-fast bacillus (AFB) staining and CSF bacterial culture were negative. Serum antinuclear antibody (ANA) profile showed positive results for dsDNA, nucleosomes and for histones antibodies. Serum antineutrophil cytoplasmic antibodies (ANCA) profile (pANCA, cANCA, anti-MPO [myeloperoxidase] antibodies), antiphospholipid antibodies, Venereal Diseases Research Laboratory (VDRL), enzyme-linked immunosorbent assay (ELISA) for human immunodeficiency virus (HIV) screening, hepatitis B surface antigen (HBsAg), hepatitis C virus (HCV) and scrub typhus antibody, were negative. Serum angiotensin-converting enzyme (ACE) level and computed tomography thorax was normal. Patient was treated with intravenous methylprednisolone 1 g daily for 5 days. Within a week, she was able to walk without support. However, bowel and bladder dysfunction took 35 days to recover. Follow-up MRI with MRA showed complete resolution of myelitis with mild reduction in spinal subarachnoid bleed and no evidence of vasculitis (Fig. 3)
Patient was on mycophenolate mofetil 500 mg b.i.d., hydroxychloroquine 100 mg b.i.d. and prednisolone 20 mg b.i.d. for 4 weeks. Steroid dose was tapered over 6 months and stopped. Now patient is taking mycophenolate mofetil 500 mg b.i.d. and hydroxychloroquine 100 mg b.i.d. without any flares for 18 months.
DISCUSSION
Acute severe back pain and headaches followed by symptoms of transverse myelopathy is usually the clinical manifestation of SSH. The causes of SSH include arteriovenous fistula, coarctation of aorta, angioma, telangiectasia, mycotic aneurysm of a spinal artery, syphilis, polyarteritis nodosa, tumors such as ependymoma, schwannoma, neurofibroma, glioblastoma multi-
forme, meningeal sarcoma) and anticoagulant therapy. Delay in diagnosis of SSH will result in poor outcome.2
On review of literature, we were able to find only few reports of SSH in patients with SLE. Most of them were due to vasculitis of spinal vessels which results in SSH, diagnosed either by digital subtraction angiography or autopsy.3 Probable cause for transverse myelitis in SLE is antiphospholipid antibody associated thrombosis of spinal vessels, which results in spinal cord ischemia or necrosis.4 However, data to support this theory has been sparse. But in our case, serum vasculitis profile, antiphospholipid antibody and MR spinal angiogram was normal. Spinal DSA was not done as patient was not willing. Hence, the cause for SSH in our case is not known.
Diminished CSF glucose level has been suggested to be associated with SLE-induced transverse myelopathy; however, spinal subarachnoid bleed may also yield this finding.2 One more finding which supports inflammatory nature of the disease process here is predominant lymphocytes in CSF.
Transverse myelitis is a very rare complication occurring in only 1 to 2% of patients with SLE.5 Other systemic autoimmune disorders associated with transverse myelitis or NMOSD are Sjögren’s syndrome (SS), primary antiphospholipid antibody syndrome, sarcoidosis and various forms of vasculitis.6 Pittock et al noted NMO-IgG seropositivity in some patients with SLE and in SS having NMO or NMOSD.7 However, autoimmune disorders had been seen equally in both NMO seropositive and negative cases.8 In our case, serum NMO antibody was negative. Spinal cord involvement in SLE is usually cervical and less often in thoracic segments. But, our case had acute transverse myelitis at conus medullaris which is a rare presentation and this type of demyelination, most likely to be clinically isolated syndrome in SLE, has good clinical improvement in more than 90% of cases.1 There is no available biomarker for predicting neuroflares in SLE; hence, identification of such markers will help in better management.
Treatment of acute SSH with transverse myelitis includes high-dose steroids and plasmapheresis followed by long-term immunosuppression. Our case had good clinical response to intravenous steroids without any further intervention.
CONCLUSION
Clinicians must be aware of the rare presentation of SLE flare-up as acute SSH and various types of demyelination in SLE. In future, identification of predicting biomarkers for neuroflares in SLE will help in better management.
REFERENCES
- Chessa E, Piga M, Floris A, Mathieu A, Cauli A. Demyelinating syndrome in SLE: review of different disease subtypes and report of a case series. Reumatismo. 2017;69(4):175-83.
- Fody EP, Netsky MG, Mrak RE. Subarachnoid spinal hemorrhage in a case of systemic lupus erythematosus. Arch Neurol. 1980;37(3):173-4.
- Tang SC, Lee CF, Lee CW, Jeng JS. Systemic lupus erythematosus flare up manifestation as cerebral and spinal subarachnoid hemorrhage. Lupus. 2011;20(11):1211-3.
- Katsiari CG, Giavri I, Mitsikostas DD, Yiannopoulou KG, Sfikakis PP. Acute transverse myelitis and antiphospholipid antibodies in lupus. No evidence for anticoagulation. Eur J Neurol. 2011;18(4):556-63.
- Kovacs B, Lafferty TL, Brent LH, DeHoratius RJ. Transverse myelopathy in systemic lupus erythematosus: an analysis of 14 cases and review of the literature. Ann Rheum Dis. 2000;59(2):120-4.
- Wingerchuk DM, Lennon VA, Lucchinetti CF, Pittock SJ, Weinshenker BG. The spectrum of neuromyelitis optica. Lancet Neurol. 2007;6(9):805-15.
- Pittock SJ, Lennon VA, de Seze J, Vermersch P, Homburger HA, Wingerchuk DM, et al. Neuromyelitis optica and non organ-specific autoimmunity. Arch Neurol. 2008;65(1):78-83.
- Nakashima I, Fujihara K, Miyazawa I, Misu T, Narikawa K, Nakamura M, et al. Clinical and MRI features of Japanese patients with multiple sclerosis positive for NMO-IgG. J Neurol Neurosurg Psychiatry. 2006;77(9):1073-5.